I feel really good about this test. I have studied a lot by studying in a group, by going over notes, by doing online worksheets, watching videos, and doing the stoichiometry section in my final review guide. After going over my quiz it seems like I only made little mistakes but actually had a decent understanding over all of these concepts. Also I think that after doing the lab in class I feel more confident in my ability to do percent yield.
Here are some videos that may help you study
Link 1
Link 2
Link 3
Monday, December 14, 2015
Getting our Quiz Back
After learning my grade I felt like I was hopeless for this unit. I thought that I had a good understanding of the concept but once receiving my grade back it showed that I had not had as good of an understanding that I had thought. But once actually getting our quiz back I had noticed that it wasn't that I had done the math section wrong but had instead either wrote out the equations wrong or balanced them wrong. If it wasn't for this I probably could have gotten a decent grade. Before our unit test I need to make sure I am confident on my skills of writing an equation and balancing it.
If you are having troubles with this here is a video that may help.
If you are having troubles with this here is a video that may help.
Sunday, December 13, 2015
Lab day 1 and 2
In this lab we added Copper (II) Chloride and water to a baby food jar. Then after we stirred the solution we placed a nail into it. After placing the nail into the solution we left it for one day. Then when we came back the next day we had to siphon off the Iron (II) Chloride leaving all the copper in the baby jar. After doing this we had to wash off the copper. Finally we have to let it dry for 1 to 2 days. I thought this was a pretty cool lab and the pre lab quiz was fairly simple because it was a question based off stoichiometry. Here are some pictures from are lab.
Stoichiometry
Google defines Stoichiometry as, "the relationship between the relative quantities of substances taking part in a reaction or forming a compound, typically a ratio of whole integers." From what we have learned in class it seems like a pretty simple concept. In some of our simpler problems we were given a specific amount of a substance and had to see how many grams of our product would be produced. In other problems we would have to find how much of a reactant we would need to make a specific amount of product. Here are a couple of examples
Here are also some videos that may help
Video 1
Video 2
Here are also some videos that may help
Video 1
Video 2
Friday, December 4, 2015
Studying all night
Today we had our unit test for chemical reactions. I was very worried that I wouldn't do good because the last test I took I didn't understand much of it. For this test I went above and beyond to study: I studied with friends, I did all the study guides, I read the chapters in the textbook, I watched a bunch of videos, and I found some more websites that helped me. I believe this studying paid off because I was able to understand most of the test. I also believe that because the test had less math it was easier to finish on time.
Some links that helped me learn the material:
Link 1
Link 2
Link 3
Some links that helped me learn the material:
Link 1
Link 2
Link 3
Reactivity Series Lab
For this unit we did a chemical reaction lab that focused on reactivity series. During this lab we combined different compounds to see if a reaction would happen then based on those reactions we were able to create are own reactivity series. I believe that this lab really helped me understand this concept. Then when we did the post lab problems I was very confused at first but then when I started getting a hang of it I was really able to understand reactivity series.
Here are two links that explains reactivity series
link 1
link 2
Here are some pictures from the lab:
Here are two links that explains reactivity series
link 1
link 2
Here are some pictures from the lab:
Sunday, November 29, 2015
Transfer of electrons: Redox
Last week we learned about redox reactions which is when electrons are transferred from the metal to the non-metal. If a species loses electrons it is said to be oxidized. If a species gains electrons it is said to be reduced. We learned that in redox single replacements the metals have changed places, metals attack metals, and reaction is based on reactivity series. Here is a link to help with redox reactions.We also learned about: synthesis, decomposition, and combustion
Synthesis is when two or more reactants combine to form one product
Decomposition is when one reactant produces two or more products
Combustion is when a hydrocarbon reacts with oxygen and the products are always CO2 and H20
Synthesis is when two or more reactants combine to form one product
Decomposition is when one reactant produces two or more products
Combustion is when a hydrocarbon reacts with oxygen and the products are always CO2 and H20
Sunday, November 22, 2015
Night before the quiz
I feel like I really understand this chapter and have been studying a lot so I feel like I will do really well on this quiz. For this quiz I went back over my notes, went over the lab we did, watched videos, learned the solubility rules, and did practice problems. I think the thing that is worrying me tomorrow is that the quiz is only 20 minutes and I usually don't do a well on timed tests.
Here are some of the videos I used to help me study:
Link 1
Link 2
Here are some of the videos I used to help me study:
Link 1
Link 2
Lab!!!!
During this lab we got to use the solubility rules to predict the precipitate of different substances. For example when my partner and I mixed KI and AgNO3 we got to predict the outcome and then see if it really happened which it did and the ending product was AgI (s). I believe this lab really helped me understand the lesson we are doing now and gave me a lot of practice figuring out molecular and net ionic equations.
Set up of the lab:
Set up of the lab:
Safety first! (Must wear goggles in this lab):
Monday, November 16, 2015
R.I.P. to my chem grade
Today we took the unit test for chem and I didn't even finish the test. I had a bunch of answers I just had to fill in because time ran out. The problem is that I studied so hard for this test and probably still failed. I studied with friends, did the practice problems online, watched videos, looked over the recent quiz, and studied the polyatomics. Even after doing this I got to the quiz and didn't know how to do a bunch of the problems.
Here were some of the links that I used:
link 1
link 2
link 3
link 4
Here were some of the links that I used:
link 1
link 2
link 3
link 4
Sunday, November 15, 2015
FINALLY GOT IN A LAB!
This week we did the formula of a chlorine lab. But before that we had to pass a pre lab quiz. For the quiz we had 3 minutes to determine the formula of a compound given the following information: the mass of the beaker, the mas of the beaker + the hydrate, and the mass of the beaker+ the contents after heating. I actually passed this quiz so I got to get into the lab in the lab we had to determine the formula of the compound zinc chloride by measuring masses involved in the reaction of zinc with hydrochloric acid. It was fun actually being able to do a lab!
Monday, November 9, 2015
After pre lab quiz and night before quiz
Again only one group ended up passing the pre-lab quiz. I studied for it but I got really nervous when taking it because it was timed and I ended up getting the problem wrong. Tomorrow is the quiz and I am pretty nervous for it. I feel like I understand it in class but then when I get to the test it totally leaves my mind. So far I have reviewed my notes and gone over the chart we have to memorize a couple of times. I will also review polyatomics and try to watch some videos that might help me.
Here are videos that might help:
Video 1
Video 2
Here are videos that might help:
Video 1
Video 2
Sunday, November 8, 2015
night before pre lab
I am very nervous for this pre lab. Last pre lab quiz only one group got to do the actual lab. For this quiz I went over the lab, looked at my notes, and also did practice problems. Although I did all of this is still do not feel that confident.
Wednesday, November 4, 2015
Mole
Today we learned about mole and how to use it. Mole is shown as 6.02 X 10 ^23. When solving problems using mole you want to write down all the given information, label all quantities, then start with what is given and decide on a path to get the answer. When learning this concept we used this diagram to help.

http://media.showme.com/files/28315/pictures/thumbs/492108/first_thumb1352935272.jpg
Today when coming into class I thought I would not be able to understand this concept but once we actually did some practice problems it started to make more sense.

http://media.showme.com/files/28315/pictures/thumbs/492108/first_thumb1352935272.jpg
Today when coming into class I thought I would not be able to understand this concept but once we actually did some practice problems it started to make more sense.
Wednesday, October 28, 2015
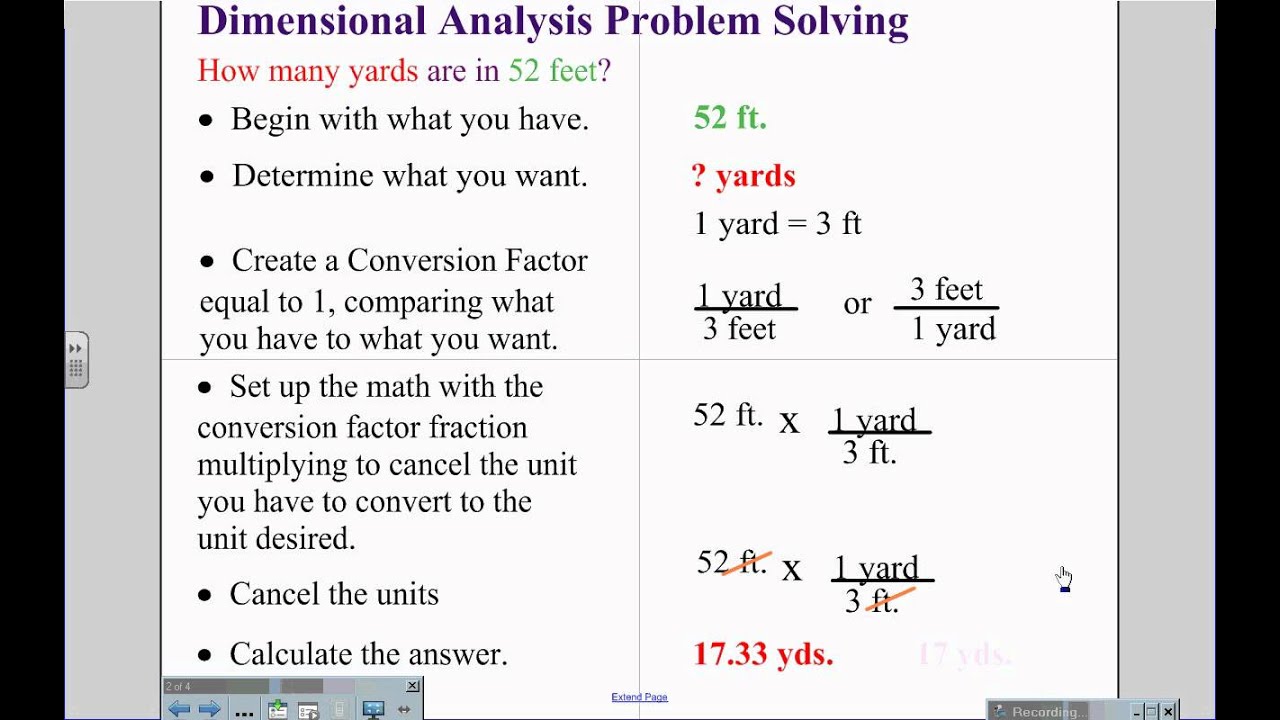
Demensional Analysis
This week we learned about demensional analysis. We used demensioanal analysis to convert one quantity to another. For example if you want to convert 8.00m to inches you have to use demensional analysis to get there. For this problem you will multiply 8.00mX 100cm/1m X 1in./2.54cm=315 in. For the most part i had a good understanding of this concept because we learned how to do most of it in trig this year . In case you need more help with dimensional analysis here are two videos:
Video 1
Video 2
Here are some examples of dimensional analysis:

http://static.prometheanplanet.com/images/resources/resource-thumbnails/thumb-nprealg-06-01-0007-diagram-thumb-lg-png.png

http://crescentok.com/staff/jaskew/isr/chemistry/factors2.gif
https://i.ytimg.com/vi/JX2ojN5FKFo/maxresdefault.jpg
Also here is a conversion table that might help:

http://cdn.rainbowresource.netdna-cdn.com/products/015024.jpg
Video 1
Video 2
Here are some examples of dimensional analysis:

http://static.prometheanplanet.com/images/resources/resource-thumbnails/thumb-nprealg-06-01-0007-diagram-thumb-lg-png.png

http://crescentok.com/staff/jaskew/isr/chemistry/factors2.gif

https://i.ytimg.com/vi/JX2ojN5FKFo/maxresdefault.jpg
Also here is a conversion table that might help:

http://cdn.rainbowresource.netdna-cdn.com/products/015024.jpg
Sunday, October 25, 2015
Pre quiz jitters
Today is the day before the quiz and I'm not sure how I feel about it yet. I understand the concepts of matter such as homogeneous and heterogeneous and concepts such as physical and chemical changes but when it comes to significant figures I get really confused. One video that helped me at least a little with significant figures was this crash course video.
Friday, October 23, 2015
Significant Figures
Yesterday in class we learned about significant numbers. This was a difficult concept to learn because you have to overlook previous math knowledge. One of the most important things we learned during this lesson was the rules of significant numbers:
In the number:
0.004004500
Video 1
Video 2
Sig Fig Song :)
In the number:
0.004004500
- The first 0 is not significant and is only there for "cosmetic purposes"
- The next two zeros are not important either and are used only to locate the decimal points
- The non zero integers are significant
- All zeros in between the non zero numbers are significant
- Significant zeros to the numbers to the right of the decimal point
Video 1
Video 2
Sig Fig Song :)
Thursday, October 1, 2015
The Test was Alright....
Today we took the unit test over Atomic Structure and Radioactivity. This test mostly focused on Daltons atomic theory, the types of radiation, how to calculate half life's and average mass equations, acids and polyatomics, fusion and fission, and we also had to be able to read graphs. I am not sure how I did on this test. I think I did okay but I could have messed up on some problems from overthinking them. I know though, that I need to go over half life questions because those were the most difficult questions for me. Although I am not sure how I did, I did a lot of studying I read the text book pages, watched videos, and focused in class.
Here is a link to a video to help with half life.
Here is a link to a video to help with half life.
Tuesday, September 29, 2015
New Lab and Learning Excel
During a lab that we started yesterday and finished today, we had 567 small square pieces of paper or "atoms" that was plain on one side and colored on the other. In this lab we put all of the squares in a cup and mixed them up. After mixing them up we separated the color squares from the plain squares. After separating them we counted the white squares or (radioactive atoms) back in the cup and removed the colored ones (decayed atoms) We did this six times. What this did was taught us about half life. Here is a link that explains what half life is. After the completion of the lab we had to graph the data in excel. Most of us have never used excel before but still we were able to understand it pretty fast.
Using Excel
Here is our data from the lab
Wednesday, September 23, 2015
Freaking out for nothing
Today we took our first quiz in the unit Atomic Structure and Radioactivity. During this section we learned about how to find the protons, neutrons, and electrons of each element and also learned the formula that helped us calculate the average atomic weight of isotopes. Before this quiz I was freaking out because I thought there were going to be very difficult answers so I study a decent amount last night and this morning right before the test. This studying paid off because I believe I ended up doing pretty well. One thing that helped me study was being able to look back on all of my notes that I took from this unit and last. These notes really help me. Also there are a couple Bohzeman videos that explain the periodic table and show you where to find different things on it. This was also very helpful. Compared to the pre-test that we took awhile ago I feel confident that I have gained more knowledge on this subject.
Bohzeman Videos can be watched here!
Bohzeman Videos can be watched here!
Tuesday, September 22, 2015
Beanium Lab
Today in class we did the beanium lab. During this lab we had to: identify the number of beanium isotopes, determine the mass of each isotope, find the percent abundance in each isotope, and calculate the average atomic mass of beanium. In order to find this we had to use a balance, a sample of beanium, and a calculator. In order to start the lab we had to count the total number of beanium atoms in the sample, which was 44. After counting them we had to separate them out into different isotopes these isotopes included: white beanium (10), black beanium (7), red beanium (5), and pinto beanium (22). After counting the isotopes we had to measure the mass of all of the atoms in that specific isotope. One thing we had to make sure we did before this is zero the balance out. Next, my lab partner and I had to calculate the average mass of this isotope. In order to do this we had to divide the number of atoms of the isotope by the total mas of all the atoms of this isotope. Then, we had to find the percentage abundance of the isotopes. To do this we had to divide the number of atoms of the certain isotope by the number of total atoms. Lastly, we had to calculate the average atomic mass of beanium. This lab is important because it taught us how to calculate the average mass of an individual isotope, the percent of abundance, and the average atomic mass of beanium.
Thursday, September 17, 2015
Atomic inference activity
Yesterday we completed an inference activity. In this activity there were covered plates with different designs and a marble. What we had to do was roll the marble around and try to infer what shape or design was without being able to see it. After a couple minutes of trying to figure out what shape it was me and my lab partner wrote down our inferences and then uncovered the plate. Most of the time we ended up being wrong. One thing that was hard about this lab was that we couldn't see what we were doing. This is comparable to Ruthfords experiment where he bounced things off the atom trying to find the structure of it.
Wednesday, September 16, 2015
Atomic theory
Today in class we learned about the atomic theory, law of conservation, and new discoveries of the atom. First, Dalton's theory stated: that elements are made up of tiny particles called atoms, all atoms of a given element are identical, the atoms of a given element are different from those of any other element, atoms of one element can combine with atoms of other elements to form compounds, atoms are indivisible in chemical processes and are not simply created or destroyed. We also learned that points number 2 and number 5 are invalid because of new information that has come about. Next, we learned about the law of constant composition. What this means is that every compound of the same species are always put together in the same order. For example, water is always 2 hydrogen's and an oxygen. Lastly, we learned about new discoveries of the atom. JJ Thomson was the person who discovered electrons by using a cathode ray to show the atoms of any element emit particles with a negative charge. This is shown through the plum pudding or chocolate chip model. Ruthford used the gold foil experiment to prove the presence of a positively charged center in an atom. Finally, the current model is the cloud model where electron location is pinpointed using probability.

http://www.humanthermodynamics.com/Atom_diagram.jpg
Atomic theory song here!
The history of the atom/Crash Course here!
Here is a website that may help with Atomic Theory here!
Atomic theory timeline here!

http://www.humanthermodynamics.com/Atom_diagram.jpg
Atomic theory song here!
The history of the atom/Crash Course here!
Here is a website that may help with Atomic Theory here!
Atomic theory timeline here!
Monday, September 14, 2015
Nomenclature
For this unit we learned about naming ionic compounds as well as acids and polyatomics. For type one you take off the ending of the anion and add -ide so MgS becomes Magnesium Sulfide. For type two we learned how to name a compound with a transition metal and non-metal. In order to do this you have to make sure you add roman numerals. For example, CuBr becomes, Copper (I) Bromide. Then for type three it is a compound between two non-metals. For this you have to remember to add the prefixes, such as penta- for five. For naming polyatomics it is mostly memorization. Lastly, naming acids if there is no oxygen in it it gets the prefix hydo- and ends in -ide. If it has an oxygen you have to look at the ending if it has the prefix -ite it changes to (root)ous acid if it ends in -ate it changes to (root)ic acid.
Overall I found this unit fairly easy. Most of it was just memorization. During this unit i have to make sure i remember most of it because we will end up using it throughout the year. In order to improve my knowledge on things such as polyatomics i can review my flashcards and look up different websites that could help me. Most of this i would have to say was my best effort, yes i can work on some things but overall i believe i understood the concepts fairly well and but in my best work.
Overall I found this unit fairly easy. Most of it was just memorization. During this unit i have to make sure i remember most of it because we will end up using it throughout the year. In order to improve my knowledge on things such as polyatomics i can review my flashcards and look up different websites that could help me. Most of this i would have to say was my best effort, yes i can work on some things but overall i believe i understood the concepts fairly well and but in my best work.
Frontier Project
During the frontier project we found plants in Eastern Deciduous Forest and Tall Grass Prairie habitat that helped with different maladies. During this project i found it difficult to find plants for each malady such as burns. I found that there are many resources online that help as well as books that can be found at the library. For my project i want people to notice how for some of the maladies i was able to find multiple plants. If i was to do this project again i most likely would start as soon as i got the project so that way i wouldn't have to fit it all in to the last two weeks. Also i would have to make sure what plants were in season because when trying to get my pictures for the project i kept looking for plants that were not even in season.
This relates to the real world because many chemists use these plants chemical properties to make the medicine we use today. Also it is good to have the knowledge of medicinal uses of plants in case they are needed in a survival situation. From here I could continue my research and pass my knowledge on to others.
This relates to the real world because many chemists use these plants chemical properties to make the medicine we use today. Also it is good to have the knowledge of medicinal uses of plants in case they are needed in a survival situation. From here I could continue my research and pass my knowledge on to others.
Thursday, August 20, 2015
introduction page
Hi im Kayla! I am co-captain of the dive team (There are only two of us) and I am also a pole vaulter! I am involved in Viking way, Stuco, NHS, Treblemaker, and chem club. I lifeguard at my neighborhood pool and I am also am the oldest of four siblings.My favorite color is blue and my favorite food is ice cream.

Subscribe to:
Posts (Atom)

















